
Chemistry, 28.05.2021 20:00 pedropaulofpedrosapp
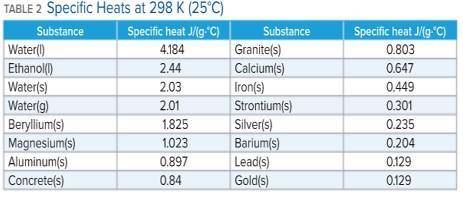
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substance absorbed 2440 J of energy. What is the specific heat of the substance? Identify the substance among those listed in Table 2.
A. the specific heat is 0.897 J/g. C, The Substance is aluminum
B. the specific heat is -0.897 J/g. C, The Substance is aluminum
C. the specific heat is 4.184 J/g. C, The Substance is water
D. there's not enough information to determine which is the substance.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substa...
Questions


Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00



Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00

Biology, 11.12.2021 01:00




Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00



