
Chemistry, 28.05.2021 19:50 queenkimm26
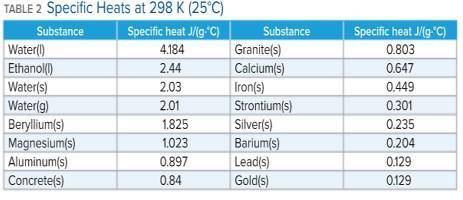
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substance absorbed 2440 J of energy. What is the specific heat of the substance? Identify the substance among those listed in Table 2.
-A. the specific heat is 0.897 J/g. C, the substance is aluminum
-B. the specific heat is -0.897 J/g. C, the substance is aluminum
-C. the specific heat is 4.184 J/g. C, the substance is water
-D. there's not enough information to determine which is the substance

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1


Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2
You know the right answer?
A 136 g sample of an unknown substance was heated from 20.0 °C to 40.0 °C. In the process the substa...
Questions


Mathematics, 18.03.2021 03:20


Chemistry, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20

Advanced Placement (AP), 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

History, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

Social Studies, 18.03.2021 03:20

Arts, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20


Mathematics, 18.03.2021 03:20

History, 18.03.2021 03:20

Health, 18.03.2021 03:20

Medicine, 18.03.2021 03:20

Mathematics, 18.03.2021 03:20



