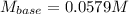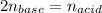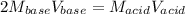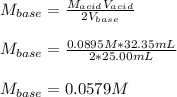Chemistry, 28.05.2021 01:10 dbzrules02
It takes 32.35 mL of a 0.0895 M hydrochloric acid solution to reach the equivalence point in the reaction with 25.00 mL of barium hydroxide. 2HCl(aq) Ba(OH)2(aq) 2H2O(l) BaCl2(aq) What is the molar concentration of the barium hydroxide solution

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
It takes 32.35 mL of a 0.0895 M hydrochloric acid solution to reach the equivalence point in the rea...
Questions

Mathematics, 06.01.2021 03:10


Business, 06.01.2021 03:10

Mathematics, 06.01.2021 03:10

Biology, 06.01.2021 03:10





History, 06.01.2021 03:10


Arts, 06.01.2021 03:10


Advanced Placement (AP), 06.01.2021 03:10

Mathematics, 06.01.2021 03:10

Arts, 06.01.2021 03:10

Mathematics, 06.01.2021 03:10


Mathematics, 06.01.2021 03:10

History, 06.01.2021 03:10