Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
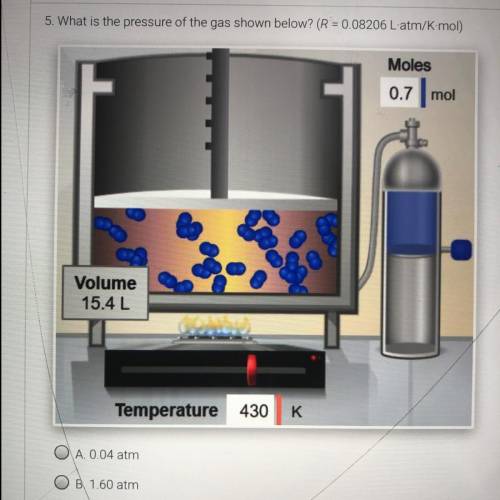
What is the pressure of the volume of the gas shown below ? (R = 0.08206 L-atm/K mol)
Answer choice...
Questions

Mathematics, 15.04.2020 06:36

Chemistry, 15.04.2020 06:36

Mathematics, 15.04.2020 06:36


Biology, 15.04.2020 06:36



Chemistry, 15.04.2020 06:36





Chemistry, 15.04.2020 06:36


SAT, 15.04.2020 06:36