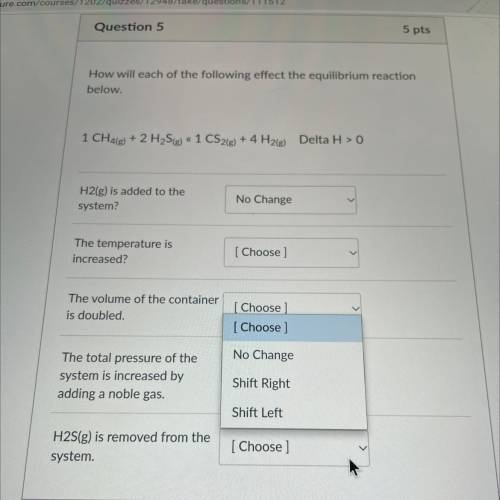
How will each of the following effect the equilibrium reaction
below.
1 CH4(g) + 2 H2S(g) « 1...

Chemistry, 27.05.2021 21:10 gerardoblk5931
How will each of the following effect the equilibrium reaction
below.
1 CH4(g) + 2 H2S(g) « 1 CS2(g) + 4 H2(g) Delta H > 0
HELPOPO

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2

Chemistry, 23.06.2019 07:00
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
Questions


Mathematics, 10.06.2021 17:00



English, 10.06.2021 17:00







Mathematics, 10.06.2021 17:00


Mathematics, 10.06.2021 17:00

English, 10.06.2021 17:00


Mathematics, 10.06.2021 17:10

Biology, 10.06.2021 17:10





