Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
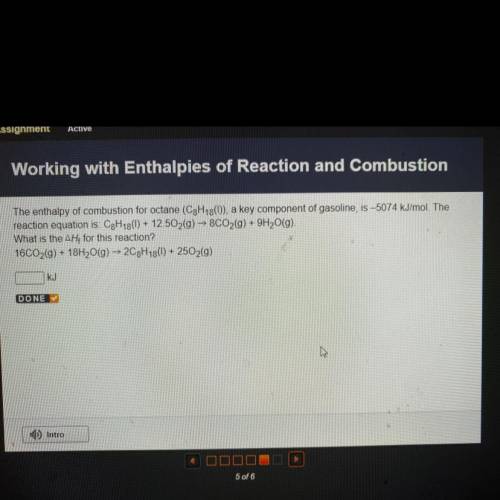
The enthalpy of combustion for octane (C3H13()), a key component of gasoline, is –5074 kJ/mol. The...
Questions

Mathematics, 08.07.2019 01:30

Social Studies, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30

History, 08.07.2019 01:30

Mathematics, 08.07.2019 01:30


Mathematics, 08.07.2019 01:30





Mathematics, 08.07.2019 01:30






Physics, 08.07.2019 01:30


Physics, 08.07.2019 01:30