
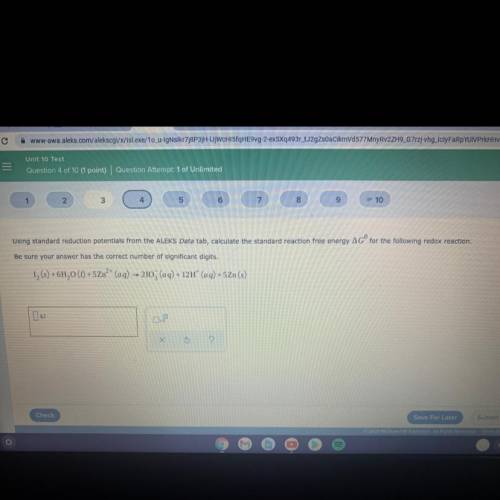
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AGº for the following redox reaction.
Be sure your answer has the correct number of significant digits.
12 (5) + 6H20 (1) + 5Zn²+ (aq) — 2103 (aq) + 12H+ (aq) +5Zn(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1


Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free en...
Questions


Computers and Technology, 12.08.2020 05:01





English, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

English, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Computers and Technology, 12.08.2020 05:01

Physics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01





Mathematics, 12.08.2020 05:01



