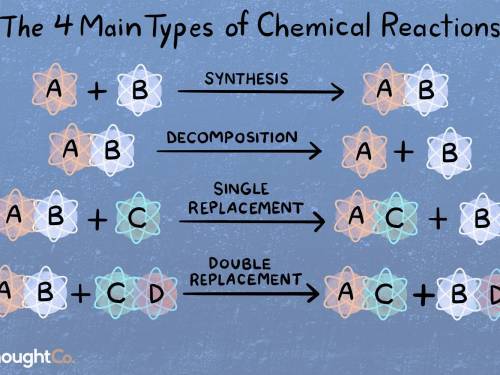
What types of reactions are these 3 chemical equations?-
*2Kl+Pb(NO3)2=PbI2+2KNO3
*2Al+3CuSO...


Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Questions


Mathematics, 21.03.2020 05:40

Physics, 21.03.2020 05:40

Mathematics, 21.03.2020 05:40





History, 21.03.2020 05:40






Mathematics, 21.03.2020 05:41


Mathematics, 21.03.2020 05:42