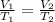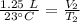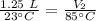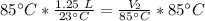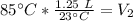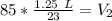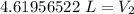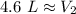Chemistry, 27.05.2021 06:40 carolinamleal04
A balloon containing helium gas has a volume of 1.25 L at room temperature (23 oC). The balloon is heated to at temperature of 85 oC . Assuming no change in pressure, what is the new volume of the balloon?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
A balloon containing helium gas has a volume of 1.25 L at room temperature (23 oC). The balloon is h...
Questions

Health, 04.07.2019 01:00




Mathematics, 04.07.2019 01:00





Computers and Technology, 04.07.2019 01:00





Mathematics, 04.07.2019 01:00


Mathematics, 04.07.2019 01:00

Biology, 04.07.2019 01:00

Mathematics, 04.07.2019 01:00

Chemistry, 04.07.2019 01:00