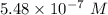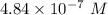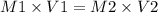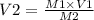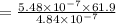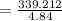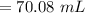A chemist must dilute 61.9 mL of 548. nM aqueous sodium carbonate (Na2CO3) solution until the concentration falls to 484. nM . He'll do this by adding distilled water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
A chemist must dilute 61.9 mL of 548. nM aqueous sodium carbonate (Na2CO3) solution until the concen...
Questions


Mathematics, 31.08.2021 01:00


Mathematics, 31.08.2021 01:00



Mathematics, 31.08.2021 01:00

Computers and Technology, 31.08.2021 01:00


History, 31.08.2021 01:00

Mathematics, 31.08.2021 01:00



Mathematics, 31.08.2021 01:00

English, 31.08.2021 01:00

Computers and Technology, 31.08.2021 01:00


Mathematics, 31.08.2021 01:00