
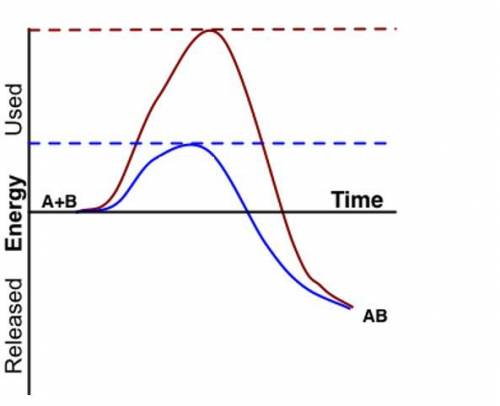
Student A determined that the red (higher) line represents the faster reaction.
Student B determined that the blue (lower) line represents the faster reaction.
Which student is correct and why? Be sure to specifically use information from the graph to support your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1

You know the right answer?
Student A determined that the red (higher) line represents the faster reaction.
Student B determine...
Questions


Mathematics, 03.11.2019 14:31






Arts, 03.11.2019 14:31

Physics, 03.11.2019 14:31

History, 03.11.2019 14:31

Mathematics, 03.11.2019 14:31

Mathematics, 03.11.2019 14:31

Mathematics, 03.11.2019 14:31


Mathematics, 03.11.2019 14:31

Social Studies, 03.11.2019 14:31

History, 03.11.2019 14:31






