
Chemistry, 26.05.2021 21:40 asenath6477
Which of the following collisions will initiate the reaction? The energy of the collision is written in kJ beside the possible orientation of the molecules.
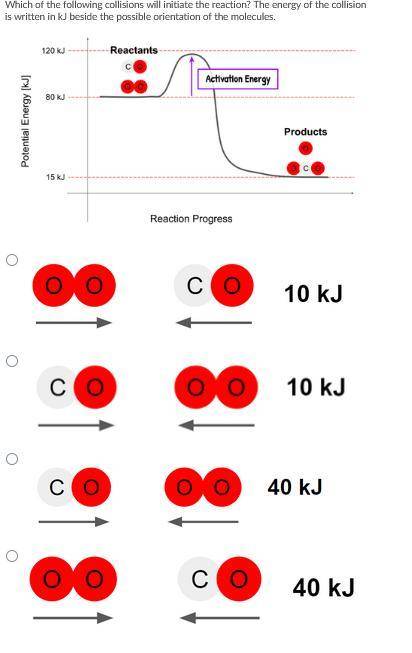

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Which of the following collisions will initiate the reaction? The energy of the collision is written...
Questions

History, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50

Chemistry, 08.12.2020 22:50

Geography, 08.12.2020 22:50

English, 08.12.2020 22:50


Mathematics, 08.12.2020 22:50

French, 08.12.2020 22:50

English, 08.12.2020 22:50




Mathematics, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50





History, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50



