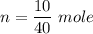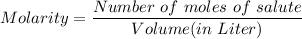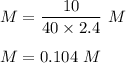Chemistry, 26.05.2021 20:30 hamilclips6805
What is the molarity of a solution prepared by dissolving 10.0 grams of NaOH in enough water to make a solution with a total volume of 2.40 liters?
O0.104 M NaOH
O0.201 M NaOH
O0.361 M NaOH
O0.412 M NaOH

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
What is the molarity of a solution prepared by dissolving 10.0 grams of NaOH in enough water to make...
Questions

Biology, 30.06.2019 14:30

English, 30.06.2019 14:30





History, 30.06.2019 14:30



Mathematics, 30.06.2019 14:30

Chemistry, 30.06.2019 14:30

Spanish, 30.06.2019 14:30


Mathematics, 30.06.2019 14:30

English, 30.06.2019 14:30



Mathematics, 30.06.2019 14:30

English, 30.06.2019 14:30