
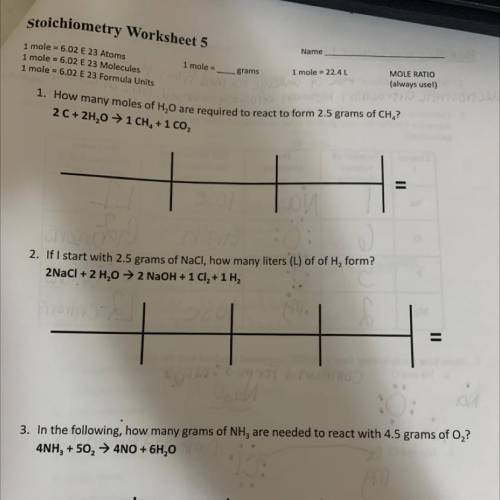
1. How many moles of H,0 are required to react to form 2.5 grams of CH,?
2 C+2H20 → 1 CH, + 1 CO2
your
GSVOS
2. If I start with 2.5 grams of NaCl, how many liters (L) of of H, form?
2NaCl + 2 H20→2 NaOH + 1 Cl2 + 1 H2
od
3. In the following, how many grams of NH3 are needed to react with 4.5 grams of 0,?
4NH3 + 502 → 4NO + 6H,
00)
To
Oci

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3


Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
1. How many moles of H,0 are required to react to form 2.5 grams of CH,?
2 C+2H20 → 1 CH, + 1 CO2
Questions



Mathematics, 10.06.2021 17:30

English, 10.06.2021 17:30


Mathematics, 10.06.2021 17:30

Biology, 10.06.2021 17:30

Mathematics, 10.06.2021 17:30

Mathematics, 10.06.2021 17:40

Geography, 10.06.2021 17:40

English, 10.06.2021 17:40


Biology, 10.06.2021 17:40

Mathematics, 10.06.2021 17:40




Biology, 10.06.2021 17:40

History, 10.06.2021 17:40

Social Studies, 10.06.2021 17:40



