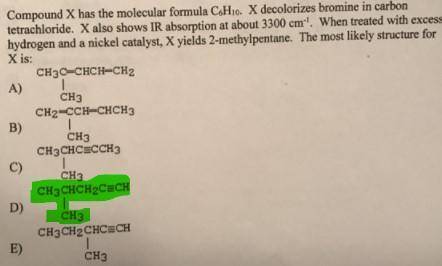Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1
You know the right answer?
Compound X has the molecular formula C6H10. X decolorizes bromine in carbon tetrachloride. X also sh...
Questions

Mathematics, 11.02.2021 19:30

History, 11.02.2021 19:30

Physics, 11.02.2021 19:30


Mathematics, 11.02.2021 19:30



Mathematics, 11.02.2021 19:30

Mathematics, 11.02.2021 19:30

History, 11.02.2021 19:30

Biology, 11.02.2021 19:30



Chemistry, 11.02.2021 19:30

Law, 11.02.2021 19:30

Mathematics, 11.02.2021 19:30

Chemistry, 11.02.2021 19:30

Spanish, 11.02.2021 19:30

Mathematics, 11.02.2021 19:30

Mathematics, 11.02.2021 19:30