
Chemistry, 26.05.2021 01:00 emilystartk
) Dinitrogen Tetroxide partially decomposes according to the following equilibrium: N2O4 (g) 2NO2 (g) A 1.00-L flask is charged with 0.400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is .

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
) Dinitrogen Tetroxide partially decomposes according to the following equilibrium: N2O4 (g) 2NO2 (g...
Questions

Mathematics, 04.07.2021 02:40

Computers and Technology, 04.07.2021 02:40


Mathematics, 04.07.2021 02:40


Mathematics, 04.07.2021 02:40


Mathematics, 04.07.2021 02:40

Mathematics, 04.07.2021 02:40

Mathematics, 04.07.2021 02:40








Mathematics, 04.07.2021 02:40

Mathematics, 04.07.2021 02:40

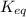 for this reaction is 1.578.
for this reaction is 1.578.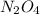 = 0.4 mol
= 0.4 mol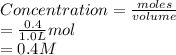
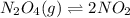
![[NO_{2}]](/tpl/images/1347/8951/53e25.png) is calculated as follows.
is calculated as follows.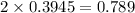
![K_{eq} = \frac{[NO_{2}]^{2}}{[N_{2}O_{4}]}\\= \frac{(0.789)^{2}}{(0.3945)}\\= 1.578](/tpl/images/1347/8951/12c3a.png)


