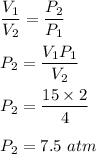Chemistry, 25.05.2021 22:30 briannagotfanz
A sample of krypton occupies 15.0 L at a pressure of 2.1 atm. Use Boyle's Law to find the pressure of the krypton when the volume is decreased to 4L.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1
You know the right answer?
A sample of krypton occupies 15.0 L at a pressure of 2.1 atm. Use Boyle's Law to find the pressure o...
Questions

Mathematics, 13.12.2021 18:50

Biology, 13.12.2021 18:50


Mathematics, 13.12.2021 19:00

Biology, 13.12.2021 19:00




SAT, 13.12.2021 19:00

Mathematics, 13.12.2021 19:00





Social Studies, 13.12.2021 19:00


English, 13.12.2021 19:00

Business, 13.12.2021 19:00

Mathematics, 13.12.2021 19:00