
Chemistry, 25.05.2021 18:00 selena5713
A sample of hydrogen gas collected by displacement of water occupied 30mL at 24 C on a day when the barometric pressure was 736t orr. What volume would the hydrogen occupy if it were dry and at STP? The vapor pressure of water at 24 C is 22.4 torr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
A sample of hydrogen gas collected by displacement of water occupied 30mL at 24 C on a day when the...
Questions



Chemistry, 29.01.2021 23:50

English, 29.01.2021 23:50

Social Studies, 29.01.2021 23:50


History, 29.01.2021 23:50


Chemistry, 29.01.2021 23:50

Mathematics, 29.01.2021 23:50

Mathematics, 29.01.2021 23:50

English, 29.01.2021 23:50


Mathematics, 29.01.2021 23:50



Biology, 29.01.2021 23:50

Mathematics, 29.01.2021 23:50


Mathematics, 29.01.2021 23:50

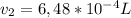
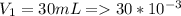
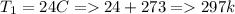
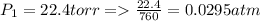
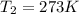
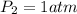
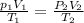
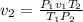
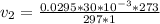
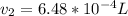 (at STP(Standard Temperature and pressure))
(at STP(Standard Temperature and pressure))

