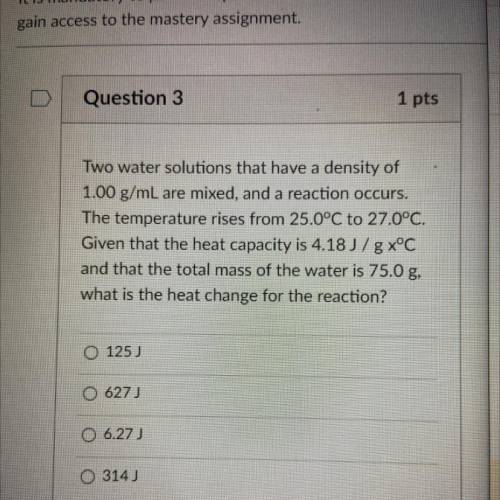
Two water solutions that have a density of
1.00 g/mL are mixed, and a reaction occurs.
The te...

Chemistry, 25.05.2021 08:50 llnapier8924
Two water solutions that have a density of
1.00 g/mL are mixed, and a reaction occurs.
The temperature rises from 25.0°C to 27.0°C.
Given that the heat capacity is 4.18 J/g x°C
and that the total mass of the water is 75.0 g,
what is the heat change for the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Questions



French, 24.05.2020 14:57


Mathematics, 24.05.2020 14:57



Mathematics, 24.05.2020 14:57

Mathematics, 24.05.2020 14:57


Biology, 24.05.2020 14:57



Social Studies, 24.05.2020 14:57

Social Studies, 24.05.2020 14:57




Mathematics, 24.05.2020 14:57

Mathematics, 24.05.2020 14:57




