Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 10:00
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
You know the right answer?
PLSSS ANSWERR
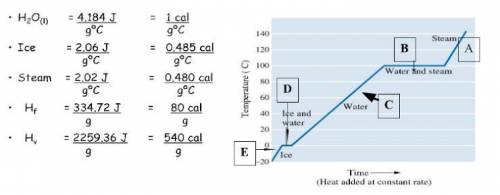
Using the above data table and graph, calculate the total energy in Joules required t...
Questions

French, 09.07.2019 20:30



Mathematics, 09.07.2019 20:30


Mathematics, 09.07.2019 20:30

Mathematics, 09.07.2019 20:30

Health, 09.07.2019 20:30


Health, 09.07.2019 20:30




English, 09.07.2019 20:30


Chemistry, 09.07.2019 20:30

Mathematics, 09.07.2019 20:30

Computers and Technology, 09.07.2019 20:30

English, 09.07.2019 20:30