
Chemistry, 25.05.2021 01:00 NetherisIsTheQueen
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion CoCl42-. Co2 (aq) is pink and CoCl42-(aq) is blue. At Low Temperature the pink color pre-dominates. At High Temperature the blue color is strong. If we represent the equilibrium as:
CoCl4^2-(aq) <--> Co2+(aq) + 4Cl-(aq)
We can conclude that:___.
1. This reaction is:___.
A. Exothermic
B. Endothermic
C. Neutral
2. When the temperature is decreased the equilibrium constant, K:.
A. Increases
B. Decreases
C. Remains the same.
3. When the temperature is decreased the equilibrium concentration of Co2:.
A. Increases
B. Decreases
C. Remains the same.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

You know the right answer?
In an aqueous chloride solution cobalt(II) exists in equilibrium with the complex ion CoCl42-. Co2 (...
Questions








Social Studies, 15.01.2020 04:31












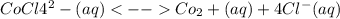
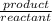 =
=![\frac{[CO^2^+][Cl^-^4]}{CoCl^2^-_4}](/tpl/images/1345/2628/18cbc.png)
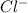 and
and  rises, and K rises as well , thus it increases .
rises, and K rises as well , thus it increases .

