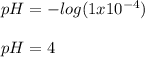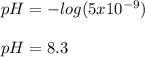Chemistry, 25.05.2021 01:00 lazavionadams81
The concentrations of hydrogen ion in two solutions are (a).1×10-4moldm and (b).5×10-9moldm. What is the pH of each solutions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3


Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
The concentrations of hydrogen ion in two solutions are (a).1×10-4moldm and (b).5×10-9moldm. What is...
Questions


Chemistry, 07.07.2019 08:30

Mathematics, 07.07.2019 08:30




History, 07.07.2019 08:30

History, 07.07.2019 08:30


History, 07.07.2019 08:30

Mathematics, 07.07.2019 08:30








Mathematics, 07.07.2019 08:30

Chemistry, 07.07.2019 08:30

![pH=-log([H^+])](/tpl/images/1345/2513/79766.png)