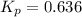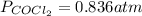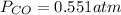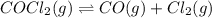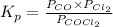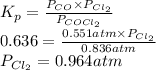The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g) + Cl2(g)
If an equilibrium mixture of the three gases in a 16.9 L container at 600K contains COCl2 at a pressure of 0.836 atm and CO at a pressure of 0.551 atm, the equilibrium partial pressure of Cl2 is atm.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


You know the right answer?
The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g)...
Questions


History, 30.01.2021 03:10


Physics, 30.01.2021 03:10


Arts, 30.01.2021 03:10



English, 30.01.2021 03:10


Mathematics, 30.01.2021 03:10



Mathematics, 30.01.2021 03:10


Mathematics, 30.01.2021 03:10


Mathematics, 30.01.2021 03:10


Chemistry, 30.01.2021 03:10

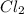 is 0.964 atm.
is 0.964 atm.