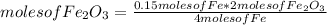Chemistry, 24.05.2021 14:00 aniecethompkins82
Using the balanced equation for the next few questions: 4 Fe(s) + 30 (9) - 2Fe2O3(s)
How many moles of Iron (11) Oxide would be produced by complete reaction of 0.15
moles of iron? (Be sure to show your work including the mole ratio you used)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
Using the balanced equation for the next few questions: 4 Fe(s) + 30 (9) - 2Fe2O3(s)
How many moles...
Questions




English, 02.07.2020 02:01


Biology, 02.07.2020 02:01



English, 02.07.2020 02:01




English, 02.07.2020 02:01

Mathematics, 02.07.2020 02:01

Health, 02.07.2020 02:01

Mathematics, 02.07.2020 02:01

Mathematics, 02.07.2020 02:01



Advanced Placement (AP), 02.07.2020 02:01