
PLEASE HELP!
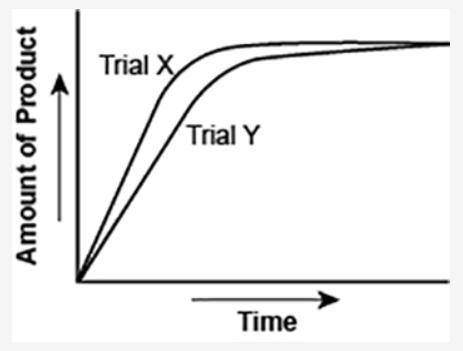
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
(I've attached the graph.)
Which of the following statements explains which trial has a lower concentration of the reactant?
a. Trial X, because the final volume of product formed is lower than Trial Y.
b. Trial X, because this reaction was initially fast and later stopped completely.
c. Trial Y, because the reaction was initially slow and later stopped completely.
d. Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
PLEASE HELP!
The graph shows the volume of a gaseous product formed during two trials of a reaction...
Questions


Business, 01.12.2019 10:31





Health, 01.12.2019 10:31

Mathematics, 01.12.2019 10:31


Computers and Technology, 01.12.2019 10:31

Biology, 01.12.2019 10:31

Computers and Technology, 01.12.2019 10:31

English, 01.12.2019 10:31

Physics, 01.12.2019 10:31









