
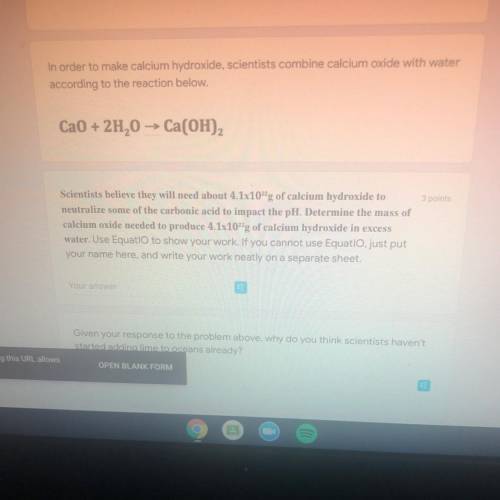
In order to make calcium hydroxide, scientists combine calcium oxide with water
according to the reaction below.
Ca0 + 2H2O -> Ca(OH)2
Scientists believe they will need about 4.1x10^22 g of calcium hydroxide to
neutralize some of the carbonic acid to impact the pH, Determine the mass of
calcium oxide needed to produce 4.1x10^22 g of calcium hydroxide in excess
water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

You know the right answer?
In order to make calcium hydroxide, scientists combine calcium oxide with water
according to the re...
Questions

Physics, 02.04.2020 01:43



Mathematics, 02.04.2020 01:44



History, 02.04.2020 01:44

Chemistry, 02.04.2020 01:44

Mathematics, 02.04.2020 01:44



Physics, 02.04.2020 01:44

Mathematics, 02.04.2020 01:44


Mathematics, 02.04.2020 01:44

Mathematics, 02.04.2020 01:44

Mathematics, 02.04.2020 01:44

Mathematics, 02.04.2020 01:44

Mathematics, 02.04.2020 01:44



