
Chemistry, 23.05.2021 22:30 jeancarlo1107
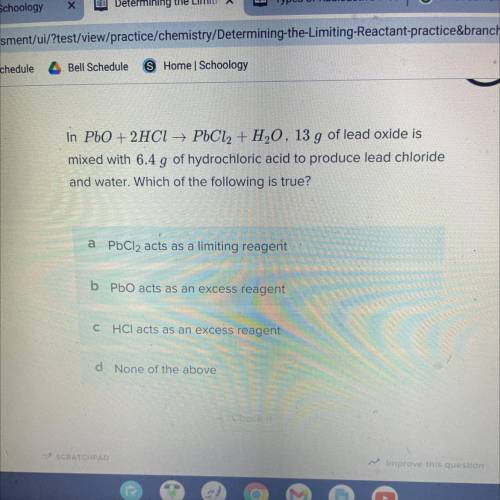
In PbO + 2HCl → PbCl2 + H20, 13 g of lead oxide is
mixed with 6.4 g of hydrochloric acid to produce lead chloride
and water. Which of the following is true?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
In PbO + 2HCl → PbCl2 + H20, 13 g of lead oxide is
mixed with 6.4 g of hydrochloric acid to produce...
Questions

Mathematics, 12.10.2019 20:30



Computers and Technology, 12.10.2019 20:30

Biology, 12.10.2019 20:30

Mathematics, 12.10.2019 20:30

Mathematics, 12.10.2019 20:30

Mathematics, 12.10.2019 20:30


Mathematics, 12.10.2019 20:30

Mathematics, 12.10.2019 20:30



Health, 12.10.2019 20:30

History, 12.10.2019 20:30



Mathematics, 12.10.2019 20:30


Physics, 12.10.2019 20:30



