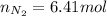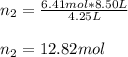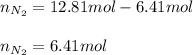Un recipiente cerrado, de 4,25 L, con tapa móvil, contiene H2S(g) a 740 Torr y 50,0°C. Se introduce en ese recipiente N2(g) a temperatura y presión constantes, de manera que el volumen final es el doble del volumen inicial. Calcular la cantidad de N2(g) en el recipiente, expresada en moles. porfi ayuda

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 04:40
Temperature is defined as a. the equivalent of heat. b. a measure of the average kinetic energy of the individual atoms or molecules composing a substance. c. how hot or cold it is. d. the total kinetic energy of the atoms or molecules composing a substance. e. none of the above is correct.
Answers: 1

Chemistry, 23.06.2019 05:30
Suppose you discovered a new element with 120 protons and 2 electrons in its outer level . i'm what group does this new element belong? what properties would you expect it to have
Answers: 1
You know the right answer?
Un recipiente cerrado, de 4,25 L, con tapa móvil, contiene H2S(g) a 740 Torr y 50,0°C. Se introduce...
Questions



Mathematics, 09.12.2020 01:00

History, 09.12.2020 01:00

History, 09.12.2020 01:00


English, 09.12.2020 01:00


Mathematics, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

Medicine, 09.12.2020 01:00

Chemistry, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00


World Languages, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00

Engineering, 09.12.2020 01:00

Mathematics, 09.12.2020 01:00