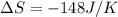AHM

Chemistry, 22.05.2021 20:00 bankscorneliuso39
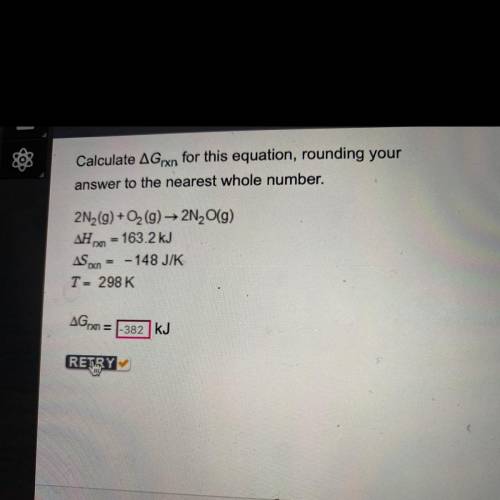
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
AHM
2N2(g) + O2(g) → 2N (9)
163.2 kJ
ASX = -148 J/K
T = 298 K
AGO
kJ

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Calculate AGrxn for this equation, rounding your
answer to the nearest whole number.
AHM
AHM
Questions


Mathematics, 17.02.2020 04:52


Mathematics, 17.02.2020 04:52


Mathematics, 17.02.2020 04:52

English, 17.02.2020 04:52



Chemistry, 17.02.2020 04:52




Mathematics, 17.02.2020 04:53

Mathematics, 17.02.2020 04:53

Biology, 17.02.2020 04:53


Social Studies, 17.02.2020 04:53


Mathematics, 17.02.2020 04:53

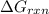 for given equation is 119.096 kJ.
for given equation is 119.096 kJ. 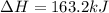 (1 kJ = 1000 J) = 163200 J
(1 kJ = 1000 J) = 163200 J