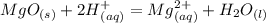Chemistry, 22.05.2021 18:10 mistycascaden
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Please let me know how you worked it out, thankyou!!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
What is the ionic equation for this reaction:
MgO (s) + 2HCl (aq) = MgCl2 (aq) + H2O (l)
Questions

History, 03.12.2020 20:30


Mathematics, 03.12.2020 20:30


English, 03.12.2020 20:30


Mathematics, 03.12.2020 20:30


Mathematics, 03.12.2020 20:30


Mathematics, 03.12.2020 20:30

Mathematics, 03.12.2020 20:30

Physics, 03.12.2020 20:30

Mathematics, 03.12.2020 20:30

Mathematics, 03.12.2020 20:30

Mathematics, 03.12.2020 20:30


Arts, 03.12.2020 20:30