

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1

Chemistry, 23.06.2019 07:30
Can you guys answer these questions i need it before 1: 00pm
Answers: 3
You know the right answer?
Propane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water....
Questions

History, 02.04.2021 21:10

Mathematics, 02.04.2021 21:10


Chemistry, 02.04.2021 21:10


Mathematics, 02.04.2021 21:10

Mathematics, 02.04.2021 21:10

Mathematics, 02.04.2021 21:10

Arts, 02.04.2021 21:10

Mathematics, 02.04.2021 21:10


Mathematics, 02.04.2021 21:10


Mathematics, 02.04.2021 21:10


Mathematics, 02.04.2021 21:10


Mathematics, 02.04.2021 21:10



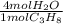 = 29.312 mol H₂O
= 29.312 mol H₂O

