
Chemistry, 21.05.2021 20:10 dari122223
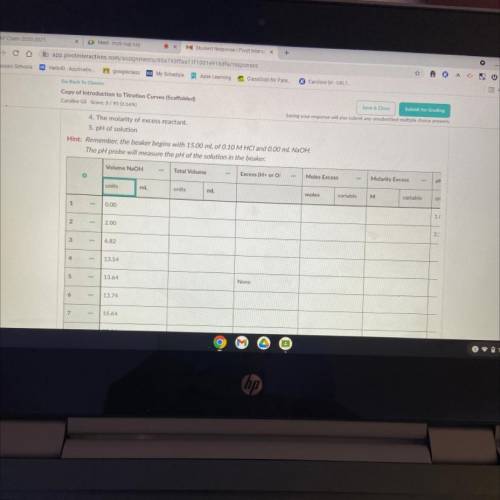
6. Now that you've predicted the equivalence point, let's predict several other points on the titration curve.
Perform calculations to predict the pH at the following points in the titration.
• 0.00 ml NaOH added
• 2.00 ml NaOH
• Half of the equivalence point volume
• 0.10 ml before the equivalence point
• Equivalence Point
• 0.10 mL after equivalence point
• 2.00 mL after the equivalence point
• 10.00 mL after equivalence point

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

You know the right answer?
6. Now that you've predicted the equivalence point, let's predict several other points on the titrat...
Questions




Computers and Technology, 22.10.2020 15:01



Social Studies, 22.10.2020 15:01


History, 22.10.2020 15:01

English, 22.10.2020 15:01




English, 22.10.2020 15:01


Mathematics, 22.10.2020 15:01

English, 22.10.2020 15:01

Mathematics, 22.10.2020 15:01

Mathematics, 22.10.2020 15:01

English, 22.10.2020 15:01



