
Chemistry, 21.05.2021 17:50 jmt13happy
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Explain your reasoning.
a. CH4 or CH3CI
b. CH3CH2CH2OH or CH3OH
c. CH3OH or H2CO

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1

Chemistry, 23.06.2019 16:30
There is a set up transformer that doubles the voltage. if the primary coil has a voltage of 10 v
Answers: 2

Chemistry, 23.06.2019 22:30
Consider the combustion of ethylene: c2h4(g)+3o2(g)→2co2(g)+2h2o(g) if the concentration of c2h4 is decreasing at the rate of 3.6×10−2 m/s , what is the rate of change in the concentration of co2? express the rate in molarity per second to two significant digits.
Answers: 1
You know the right answer?
In each pair of compounds, pick the one with the higher vapor pressure at a given temperature. Expla...
Questions

Geography, 04.03.2021 17:40

Social Studies, 04.03.2021 17:40

Social Studies, 04.03.2021 17:40



Mathematics, 04.03.2021 17:40

Mathematics, 04.03.2021 17:40






Physics, 04.03.2021 17:40

Social Studies, 04.03.2021 17:40


Mathematics, 04.03.2021 17:40


Mathematics, 04.03.2021 17:40


History, 04.03.2021 17:40

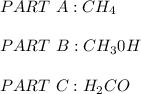
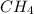 because it is non-polar, while
because it is non-polar, while 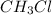 is polar.
is polar. 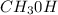 .
.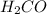 does not show hydrogen due to the increased vapor pressure
does not show hydrogen due to the increased vapor pressure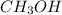 bonding.
bonding.

