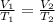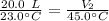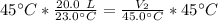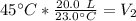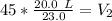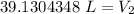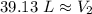Chemistry, 21.05.2021 06:10 aschool2000
A balloon contains 20.0 L of helium in the morning when the temperature is
23.0°C. By afternoon, the temperature has increases to 45.0°C. What is the new
volume of the balloon? *
O 21.49 L
39.13 L
0 0.02 L

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
A balloon contains 20.0 L of helium in the morning when the temperature is
23.0°C. By afternoon, th...
Questions

Mathematics, 07.05.2021 17:30

Advanced Placement (AP), 07.05.2021 17:30

Mathematics, 07.05.2021 17:30

Chemistry, 07.05.2021 17:30

English, 07.05.2021 17:30

Mathematics, 07.05.2021 17:30


World Languages, 07.05.2021 17:30




Mathematics, 07.05.2021 17:30



English, 07.05.2021 17:30

Mathematics, 07.05.2021 17:30

Mathematics, 07.05.2021 17:30

Mathematics, 07.05.2021 17:30


English, 07.05.2021 17:30