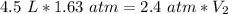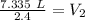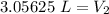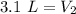Chemistry, 20.05.2021 23:50 joyelewis58
A sample of neon gas occupies 4.5 L of gas at 1.63 atm. What will be the volume if the pressure changes to 2.4 atm

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
A sample of neon gas occupies 4.5 L of gas at 1.63 atm. What will be the volume if the pressure chan...
Questions


Social Studies, 19.03.2021 03:00

Mathematics, 19.03.2021 03:00

History, 19.03.2021 03:00


Mathematics, 19.03.2021 03:00


Mathematics, 19.03.2021 03:00


English, 19.03.2021 03:00


Mathematics, 19.03.2021 03:00

Mathematics, 19.03.2021 03:00

Mathematics, 19.03.2021 03:00


Chemistry, 19.03.2021 03:00

Health, 19.03.2021 03:00