
Chemistry, 20.05.2021 07:10 lindseylewis313
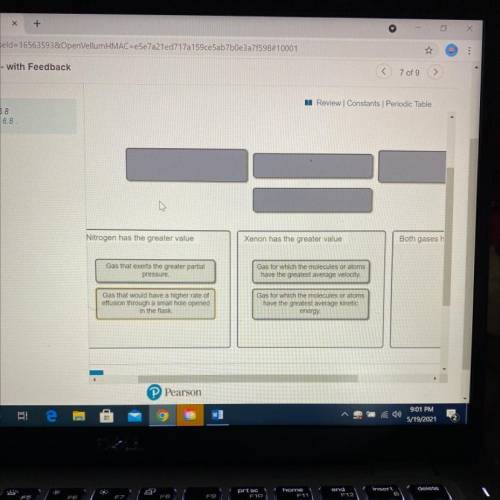
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
Sort the conditions based on the gas described.
Drag the appropriate items to their respective bins.

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:02
Which of the following provides support and protection for many insects a) muscle b) skeleton c) spinal cord d) exoskeleton
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
Sort t...
Questions

Mathematics, 03.12.2019 04:31

Mathematics, 03.12.2019 04:31

English, 03.12.2019 04:31

Mathematics, 03.12.2019 04:31


Mathematics, 03.12.2019 04:31



Biology, 03.12.2019 04:31



Mathematics, 03.12.2019 04:31


Mathematics, 03.12.2019 04:31



Biology, 03.12.2019 04:31

Mathematics, 03.12.2019 04:31




