
Chemistry, 20.05.2021 01:30 idjfjcjs584
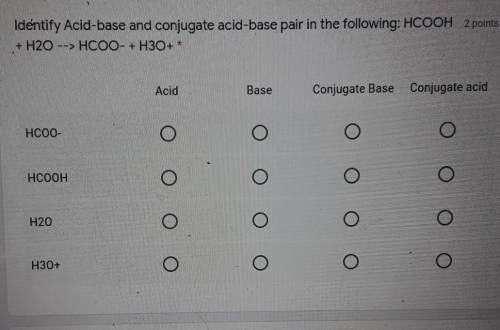
Identify Acid-base and conjugate acid-base pair in the following: HCOOH + H20 --> HCOO + H3O+ *

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Identify Acid-base and conjugate acid-base pair in the following: HCOOH + H20 --> HCOO + H3O+ *...
Questions




Advanced Placement (AP), 27.03.2020 23:20

English, 27.03.2020 23:20



English, 27.03.2020 23:20

SAT, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20


English, 27.03.2020 23:20

History, 27.03.2020 23:20



History, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20

Mathematics, 27.03.2020 23:20


History, 27.03.2020 23:20



