Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
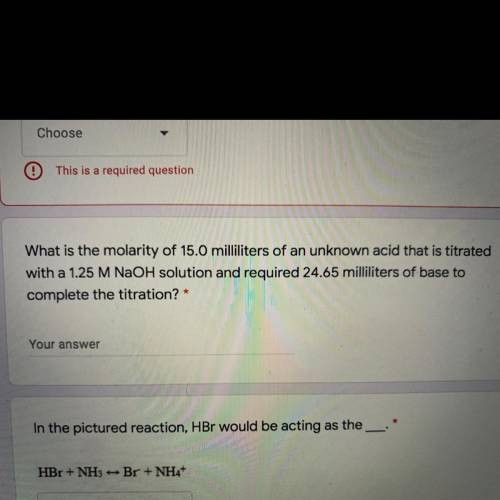
What is the molarity of 15.0 milliliters of an unknown acid that is titrated
with a 1.25 M NaOH sol...
Questions

Mathematics, 01.12.2020 18:20

Mathematics, 01.12.2020 18:20


Mathematics, 01.12.2020 18:20

English, 01.12.2020 18:20

History, 01.12.2020 18:20

Mathematics, 01.12.2020 18:20

Mathematics, 01.12.2020 18:20

Biology, 01.12.2020 18:20

English, 01.12.2020 18:20


English, 01.12.2020 18:20

Mathematics, 01.12.2020 18:20

Spanish, 01.12.2020 18:20



Biology, 01.12.2020 18:20


Mathematics, 01.12.2020 18:20