
Chemistry, 19.05.2021 06:50 bernadetteindre6650
The graph below shows the titration curve that results when 100 mL of 0.0250 M acetic acid is titrated with 0.100 M NaOH. Which of the following indicators is the best choice for this titration?
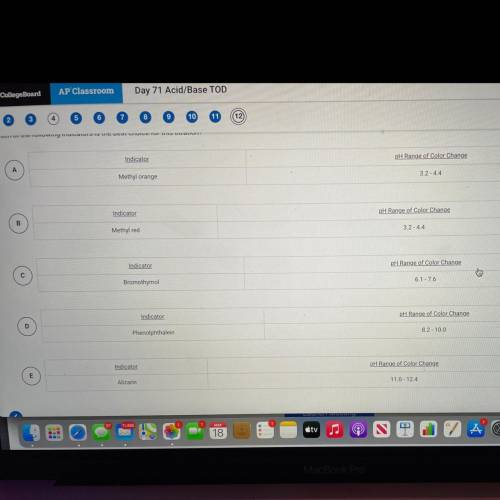

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
The graph below shows the titration curve that results when 100 mL of 0.0250 M acetic acid is titrat...
Questions



Social Studies, 27.07.2019 09:30



History, 27.07.2019 09:30

Mathematics, 27.07.2019 09:30


Biology, 27.07.2019 09:30


Spanish, 27.07.2019 09:30

Geography, 27.07.2019 09:30




Biology, 27.07.2019 09:30



Arts, 27.07.2019 09:30

History, 27.07.2019 09:30



