
Chemistry, 18.05.2021 22:50 lorenavh81
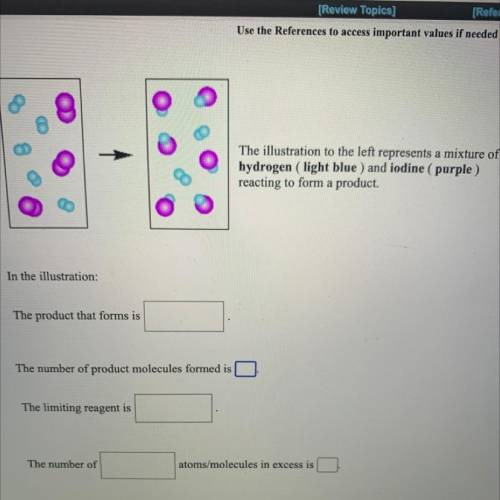
The illustration to the left represents a mixture of
hydrogen ( light blue ) and iodine (purple)
reacting to form a product.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1
You know the right answer?
The illustration to the left represents a mixture of
hydrogen ( light blue ) and iodine (purple)
Questions

Mathematics, 07.02.2021 03:10


Chemistry, 07.02.2021 03:10

Mathematics, 07.02.2021 03:10

Mathematics, 07.02.2021 03:10

World Languages, 07.02.2021 03:10

Health, 07.02.2021 03:20


History, 07.02.2021 03:20


Mathematics, 07.02.2021 03:20





History, 07.02.2021 03:20

Biology, 07.02.2021 03:20

Mathematics, 07.02.2021 03:20




