
Chemistry, 18.05.2021 22:40 ashled7789
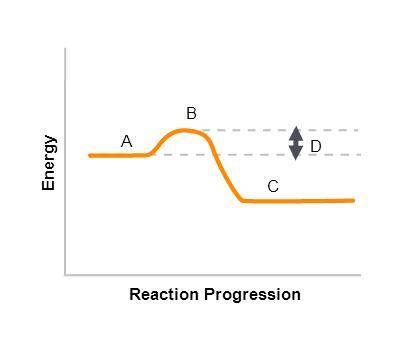
On the graph:
A represents the A)energy of reactants, B)energy of products, C)activation energy, D)activated complex .
B represents the A)energy of reactants, B)energy of products, C)activation energy, D)activated complex .
C represents the A)energy of reactants, B)energy of products, C)activation energy, D)activated complex .
D represents the activation energy .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
On the graph:
A represents the A)energy of reactants, B)energy of products, C)activation energy, D)...
Questions




Arts, 06.12.2020 03:10




Mathematics, 06.12.2020 03:10

Chemistry, 06.12.2020 03:10


Biology, 06.12.2020 03:10

Mathematics, 06.12.2020 03:10


History, 06.12.2020 03:10


Biology, 06.12.2020 03:10






