
Chemistry, 18.05.2021 21:10 marahsenno
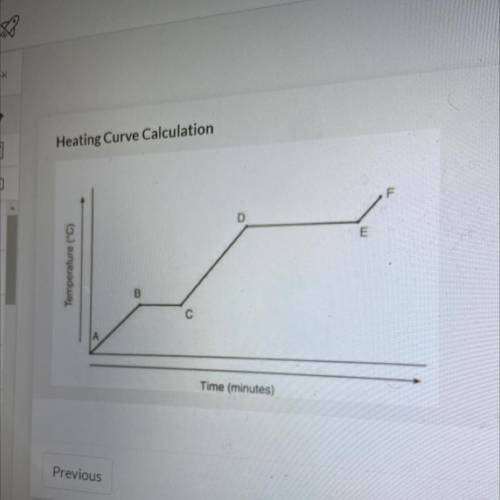
Referencing the figure to the left, how much energy in J would be
needed to change 59 g of ice at -22.75 °C into steam at 128.65 °C
Important numbers/equations:
q = Cpm: AT
Specific Heat of Water (liquid) = 4.18 J/go°C
Specific Heat of Water (solid) = 2.06 J/g•°C
Specific Heat of Water (gas) = 2.01 J/g °C
Molar Heat of Fusion of water: 6009 J/mol
Molar Heat of Vaporization for water: 40790 J/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2
You know the right answer?
Referencing the figure to the left, how much energy in J would be
needed to change 59 g of ice at -...
Questions








Geography, 27.09.2019 16:10





Geography, 27.09.2019 16:10







Geography, 27.09.2019 16:10



