
Chemistry, 18.05.2021 20:30 Nismo3501037
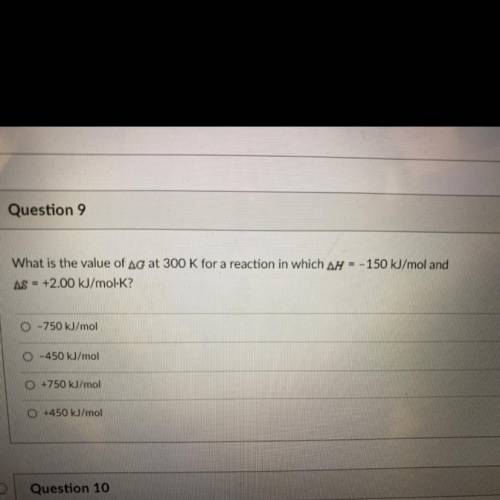
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and AS = +2.00 kJ/mol-K?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1
You know the right answer?
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and
AS = +2.00 kJ/mol-K?...
Questions

Chemistry, 08.10.2019 07:00


Mathematics, 08.10.2019 07:00



History, 08.10.2019 07:00

Mathematics, 08.10.2019 07:00


Mathematics, 08.10.2019 07:00

Social Studies, 08.10.2019 07:00



Social Studies, 08.10.2019 07:00


Spanish, 08.10.2019 07:00

Chemistry, 08.10.2019 07:00

Chemistry, 08.10.2019 07:00



History, 08.10.2019 07:00



