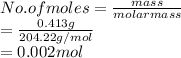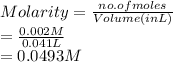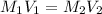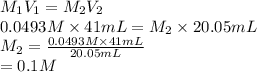Chemistry, 18.05.2021 19:20 christinamonte122
413 mg of dried KHP is dissolved in 41 mL of distilled water and titrated with potassium hydroxide (KOH). If it took 20.05 mL of KOH to reach the endpoint, determine the concentration of KOH. Show calculations.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 19:30
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1
You know the right answer?
413 mg of dried KHP is dissolved in 41 mL of distilled water and titrated with potassium hydroxide (...
Questions



History, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

French, 14.04.2021 14:00


Mathematics, 14.04.2021 14:00

English, 14.04.2021 14:00

Chemistry, 14.04.2021 14:00

Biology, 14.04.2021 14:00

English, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00


English, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Mathematics, 14.04.2021 14:00

Physics, 14.04.2021 14:00