
Chemistry, 18.05.2021 17:50 Tonyang1742
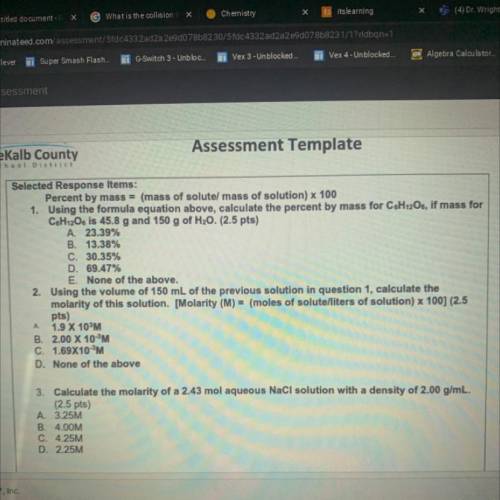
Percent by mass = (mass of solutel mass of solution) x 100
1. Using the formula equation above, calculate the percent by mass for C&H12O6, if mass for
C6H12Os is 45.8 g and 150 g of H20. (2.5 pts)
A. 23.39%
B. 13.38%
C. 30.35%
D. 69.47%
E. None of the above.
2. Using the volume of 150 mL of the previous solution in question 1, calculate the
molarity of this solution. [Molarity (M) = (moles of solute/liters of solution) x 100] (2.5
pts)
1.9 X 10%M
B. 2.00 X 10 M
C. 1.69X10M
D. None of the above
A
3. Calculate the molarity of a 2.43 mol aqueous NaCl solution with a density of 2.00 g/mL.
(2.5 pts)
A 3.25M
B. 4.00M
C. 4.25M
D. 2.25M

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Percent by mass = (mass of solutel mass of solution) x 100
1. Using the formula equation above, cal...
Questions






Mathematics, 18.09.2019 18:10

Mathematics, 18.09.2019 18:10


Social Studies, 18.09.2019 18:10


History, 18.09.2019 18:10



History, 18.09.2019 18:10

Social Studies, 18.09.2019 18:10


History, 18.09.2019 18:10



Mathematics, 18.09.2019 18:10



