
Chemistry, 18.05.2021 17:40 aahneise02
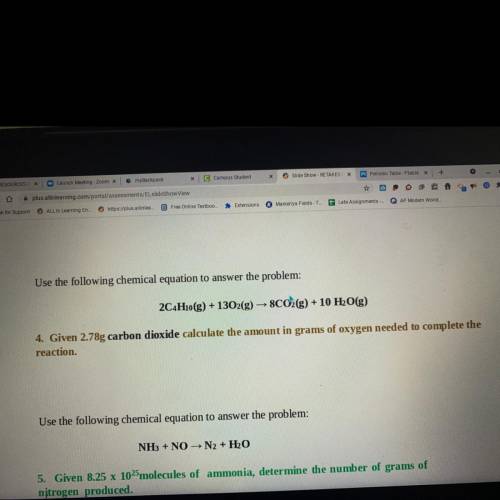
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g)
4. Given 2.78g carbon dioxide calculate the amount in grams of oxygen needed to complete the
reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g...
Questions

Biology, 20.07.2019 14:00



Health, 20.07.2019 14:00



Mathematics, 20.07.2019 14:00

English, 20.07.2019 14:00


Mathematics, 20.07.2019 14:00


Computers and Technology, 20.07.2019 14:00



Business, 20.07.2019 14:00


English, 20.07.2019 14:00



Physics, 20.07.2019 14:00



