
Chemistry, 18.05.2021 14:20 lilianjoyful
PLEASE HELP TIMED TEST
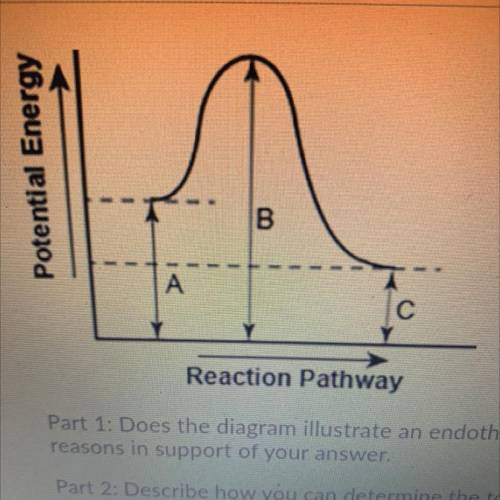
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give
reasons in support of your answer.
Part 2: Describe how you can determine the total change in enthalpy and activation
energy from the diagram and if each is positive or negative.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
PLEASE HELP TIMED TEST
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction...
Questions


Biology, 09.05.2021 07:50


Mathematics, 09.05.2021 07:50

History, 09.05.2021 07:50

Physics, 09.05.2021 07:50

Mathematics, 09.05.2021 07:50

Mathematics, 09.05.2021 07:50



Mathematics, 09.05.2021 07:50


Social Studies, 09.05.2021 07:50





Mathematics, 09.05.2021 07:50

Biology, 09.05.2021 07:50

English, 09.05.2021 07:50



