0 0
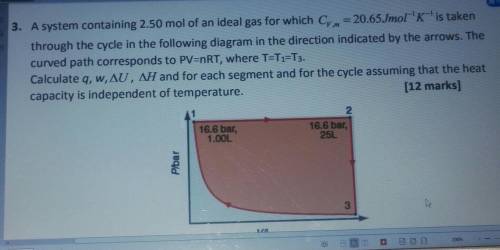
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
t...

Chemistry, 17.05.2021 23:10 pablohc200021
0 0
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
through the cycle in the following diagram in the direction indicated by the arrows. The
curved path corresponds to PV=nRT, where T=T1=T3.
Calculate q, w, AU, AH and for each segment and for the cycle assuming that the heat
capacity is independent of temperature.
(12 marks]
2.
16.6 bar,
1.00L
16.6 bar,
25L
Plbar
یا
3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Questions

Mathematics, 18.10.2019 18:30


Biology, 18.10.2019 18:30

Mathematics, 18.10.2019 18:30




Chemistry, 18.10.2019 18:30



Physics, 18.10.2019 18:30


History, 18.10.2019 18:30

Mathematics, 18.10.2019 18:30

Mathematics, 18.10.2019 18:30



Physics, 18.10.2019 18:30




