
Chemistry, 17.05.2021 22:30 itsyagirl11076
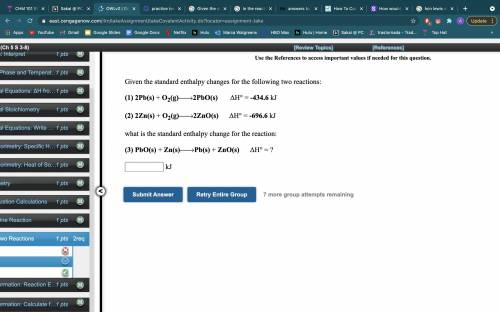
Given the standard enthalpy changes for the following two reactions:
(1) 2Pb(s) + O2(g)2PbO(s). ΔH° = -434.6 kJ
(2) 2Zn(s) + O2(g)2ZnO(s).ΔH° = -696.6 kJ
what is the standard enthalpy change for the reaction:
(3) PbO(s) + Zn(s)Pb(s) + ZnO(s).ΔH° = __kJ

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Lithium diisopropylamide [(ch3)2ch]2nli, referred to as lda, enjoys many uses as a strong base in synthetic organic chemistry. it is customarily prepared by the reaction of diisopropylamine [(ch3)2ch]2nh with butyllithium. draw the products of the reactions in the appropriate boxes and select the acid, base, conjugate acid, and conjugate base. be sure to answer all parts.
Answers: 2



Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
Given the standard enthalpy changes for the following two reactions:
(1) 2Pb(s) + O2(g)2PbO(s). ΔH°...
Questions

Biology, 05.10.2020 14:01


English, 05.10.2020 14:01

Health, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01


History, 05.10.2020 14:01

Chemistry, 05.10.2020 14:01


Physics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01




Mathematics, 05.10.2020 14:01

History, 05.10.2020 14:01


History, 05.10.2020 14:01

History, 05.10.2020 14:01



