
Chemistry, 17.05.2021 22:00 stormserena
Please help!
Samira watched a demonstration in which a scientist combined two substances in a sealed container and analyzed the results. The ending substances did not look the same as the starting substances.
Samira was given a diagram with the repeating groups of atoms that make up the starting substances, plus information about the properties of the starting and ending substances. She created a model of the repeating groups of atoms that might make up the two ending substances.
A.) Does her model correctly show why the properties of the ending substances are different from the properties of the starting substances?
B.) Describe what could be correct or incorrect about her model
Look at the diagram below then write your answer to question 13 part "A" and "B".
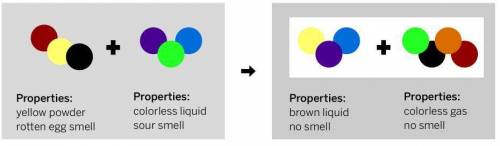

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3


Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Please help!
Samira watched a demonstration in which a scientist combined two substances in a seale...
Questions

Mathematics, 16.07.2019 20:00

Mathematics, 16.07.2019 20:00



Mathematics, 16.07.2019 20:00






Mathematics, 16.07.2019 20:00


Mathematics, 16.07.2019 20:00


History, 16.07.2019 20:00

Mathematics, 16.07.2019 20:00

Mathematics, 16.07.2019 20:00


Mathematics, 16.07.2019 20:00

World Languages, 16.07.2019 20:00



